By Marian Warner, Biotechnology ‘21
Author’s Note: I chose the subject of gene drives for UWP 104E (writing in science class) because I found it personally interesting and wanted to learn more about its controversy. The more I did research on the subject throughout the quarter the more I realized how much is unknown by the general public. The mechanism is a bit complex, so there are not many sources that attempt to explain it to a generalized audience. I hope this paper does a good job of helping those unfamiliar with gene drives become interested and gain a grasp on the scientific backing behind it. I also hope it aids them in forming an informed opinion on the subject or gives them an idea of what further questions they may want to ask.
Malaria, a mosquito-borne disease, has been a stubborn, unrelenting issue despite the established traditional methods used to combat it. Because the female mosquito in the Anopheles genus is the intermediary host transmitting the infectious agent, Plasmodium parasites, from an infected to a non-infected person, current preventative efforts mainly target the mosquitoes by dispersing insecticide-treated bed nets, insecticide for indoor use, and occasional environmental control by destroying larval habitats [1]. For infected individuals, there are treatment options such as antimalarial drugs that can suppress infections, but these target the Plasmodium parasites instead. Although these efforts have helped slow the spread of malaria, it still affected 229 million people and killed an estimated 409,000 in 2019 [2]. Fortunately, scientists such as Andrea Beaghton from Imperial College London have developed a promising strategy using genetically modified mosquitoes to rapidly spread the male-biased sex determination genes in order to exponentially decrease the amount of breeding in a population, which has proven to be effective in completely wiping out populations of malaria-carrying mosquitos in as little as 30 generations in a laboratory setting [3]. The complete collapse of malaria-carrying mosquito populations due to using this strategy in the wild would efficiently lead to the eradication of malaria.
The Role of Selfish Genetic Elements
The strategy works by utilizing a gene drive, a technique that spreads a gene throughout a population at an abnormally fast rate. Usually, any given copy of a gene has a 50 percent chance of being passed down to one’s offspring. This occurs because each diploid organism, such as a human or an insect, carries two different alleles, or variations, of a given gene. One allele is inherited from each of that organism’s parents.
When it comes to a gene drive, however, the allele in question is almost always inherited. Scientists use a naturally occurring selfish genetic element to propel gene drives. Selfish genetic elements, or selfish genes, are alleles that convert the other inherited allele into a copy of itself. In this scenario, the gene no longer has a 50 percent chance of being passed down, but instead nearly a 100 percent chance [Figure 1].
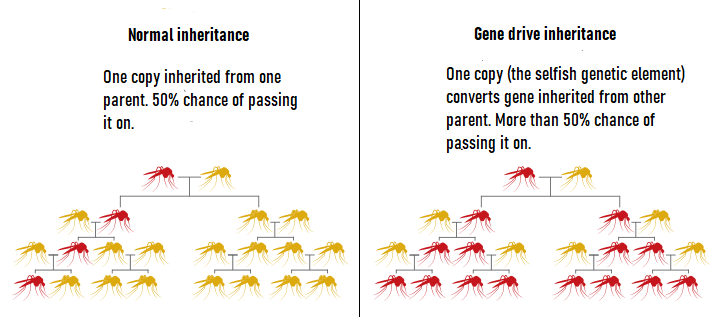
Figure 1
The Mechanism Behind the Selfish Genetic Element
To convert the other allele inherited into a replica of the selfish gene, the selfish gene exploits a natural process the genome uses to repair itself, known as homology driven repair, allowing harmful genes to bypass the rules of natural selection. The mechanism works by encoding a pair of biological scissors, an endonuclease, that cut DNA at the position of the second allele. Once the endonuclease slices open a segment of DNA, the DNA is more unstable and gets chewed back, resulting in the allele being lost. The cell registers the damage and repairs the region by copying and pasting the DNA sequence of the selfish genetic element into the spot where the naturally occurring allele was previously, resulting in both alleles being that of the selfish gene [4]. The only reason why the mechanism doesn’t work 100 percent of the time in practice is because of occasional issues the endonuclease has with recognizing the target allele [5].
The Anopheles Gambiae Gene Drive
With new powerful genome editing technologies, scientists can choose any gene to be a selfish genetic element. Many have focused on trying to identify and genetically modify an allele that could wipe out the Anopheles gambiae population, the most common mosquito species involved with the spread of malaria. However, this technique is not as simple as it seems at first glance. Trying to spread a deadly allele would not work, because the mosquitoes need to be alive and reproducing to spread it. Spreading an allele that makes mosquitoes weaker would not work either. For example, trying to eradicate their ability to fly could help suppress a population briefly, but the chances of mosquitoes forming resistance to the mechanism of this gene drive due to natural selection is high.
Instead, scientists have decided to target genes that are involved with sex determination, to make the population spread genes that reduce female survivorship. Under this gene drive, females are born with intersex mouth parts that do not allow them to feed, and therefore die relatively quickly. Targeting females is beneficial because they are the only ones capable of transmitting malaria. Additionally, as the population becomes increasingly more male-dominated, chances of reproduction become slimmer, and fewer mosquitos are born. The specific sex-determining genes for this are unlikely to invoke evolved resistance, because any changes to a pathway as specific as the sex determination pathway will most likely result in detrimental effects on the mosquito, preventing the spread of the mutations by natural selection [3]. As the gene drive rapidly spreads throughout the population, the number of malaria cases would rapidly decrease. Compared to traditional methods, the strategy would be incredibly efficient and a low cost, but there is a potential for unknown side effects, leading many to believe that using traditional methods is the best option for now.
Potential Consequences of Traditional Methods and Gene Drives
Despite the efficiency gene drives are thought to have, sceptics often argue that the current methods used to control malaria have a much lower risk of adverse side effects. Experts have approved pyrroles and pyrethroids as insecticides for mosquito bed nets because of their relatively low consequences on human health. However, mosquitos are now evolving resistance to pyrethroids. To reduce the odds of mosquitoes becoming resistant, many bed nets include multiple insecticides. However, there is not yet any evidence that these nets work in regions that already have high levels of pyrethroid resistance [6]. Similarly, Plasmodium parasites have also been found to harbor drug resistance to antimalarial drugs. Partial resistance to the drug artemisinin has already been detected in over five percent of some Plasmodium populations, and several other types of drug resistance have been detected as well. As of now, insecticides and antimalarial drugs can continue to be used effectively, but resistance to them must be closely monitored by collecting data on malarial drug treatment cases and looking for molecular markers of resistance in natural populations to prevent these methods from becoming useless in the future [1].
Many scientists agree that gene drives should be further studied as they currently have potential for more concerning side effects than traditional methods. One example of a significant concern is the unknown effect on the food chain from eliminating the A. gambiae populations [7]. So far, studies show that few animals rely solely on A. gambiae as a food source, so many experts believe the chance of negative environmental impacts are slim although there may always be the potential for side effects that were not studied in a specific sub-population or environmental niche [8]. Another concern is the potential for unethical uses with this new technology [7]. If, for example, someone releases a gene drive before enough research has been done and before it has been approved by a regulatory agency, serious environmental consequences could take place. Therefore, laws should be put in place to prevent such a thing from happening. Jim Thomas, a member of the Action Group on Erosion, Technology and Concentration says, “So far, all the proposals around gene drives are things like voluntary ethics codes and agreements between funders. They’re not binding in any way, so to what extent they can be enforced and who would be liable in the event of a problem — there’s none of that” [7]. Kevin Esvelt, one of the researchers who helped engineer the first gene drive, agrees that gene drive technology development could lead to consequences. “This isn’t just going to be about malaria,” Esvelt said. “This is potentially going to be something any individual who can make a transgenic fruit fly could build to edit all the fruit flies” [9].
The Costs of Traditional Methods and Gene Drives
Despite potential ethical and environmental concerns about the technology, the cost of gene drive research and implementation is overall lower than the cost of traditional methods and would save money in the long run. Comparatively, the cost of producing and dispersing nets, insecticides, and drugs each year is more expensive than developing and releasing a successful gene drive. The World Health Organization estimated that about $6.8 billion in resources for malaria prevention was needed in 2020, and that the cost will continue rising each year by an estimated additional $720 million [2]. As long as there is no complete way to prevent malaria, it is unlikely for the annual cost of these developments to disappear any time soon.
Unlike traditional methods, gene drive research has the potential to eradicate malaria completely and thereby curb all expenses involved in malaria research and malaria equipment dispersal. The Bill and Melinda Gates Foundation contributes a majority of grants going into gene drive research, donating about $7.4 million in grants in 2020. This funding has been going towards furthering promising research on gene drives and studies on the environmental effects of gene drives [10]. Ongoing research in future years may continue to require similar amounts of funding to the amount from current grants. However, this price is relatively small considering how much funding goes into malaria prevention and control annually, as well as the potential for a gene drive to completely curb the need for future funding of any kind. The cost of real life implementation is thought to be negligibly small, due to the process involving the release of only a small population of mosquitoes into the wild.
Efficiency of Traditional Methods and Gene Drives
Although cost is a big factor, the main reason for the huge support of gene drive research is the evidence pointing to a gene drive being a much more effective method than current traditional strategies. Current strategies such as insecticide use and drug use have not led and will unlikely lead to the elimination of malaria. Data suggests an overall trend towards fewer malaria cases likely due to traditional methods currently in place [1]. However, a full eradication of malaria around the world is the ultimate goal. Despite prevalent insecticide use and mosquito population control, there is still always a chance of a deadly mosquito bite in areas hard hit by malaria. Deadliness is especially the case when the issue of drug resistance pertains in Plasmodium. Mutations involved in partial drug resistance have already been detected in Plasmodium, and the more drugs continue to be used, the more likely resistance will continue to develop. Overall, insecticides can only be somewhat effective and cases of treatment failure are on the rise [1].
Gene drives, on the other hand, have been incredibly promising when it comes to efficiency. In the study by Beaghton, when the gene drive allele was released in a caged population and only made up 2.5 percent of the population, the entire population was predicted to crash in at least 30 generations [3]. Some models predict that even less mosquitos would need to be released into the wild in a real life scenario. One predicted that the release of just 500 gene drive mosquitoes could result in the complete collapse of a targeted mosquito species population in the timeframe of eight years [11]. Further mathematical models may be used in the future to calculate the optimal percent of the genetically modified mosquitoes that could be released to wipe out the population in the shortest timeframe feasible. From there, more gene drives targeting the other species of malaria-carrying mosquitos could be released, which would lead to a complete eradication of malaria. For now, scientists must continue to study the safety of gene drives by studying them in the lab, computationally, and perhaps in small contained real life settings. Additionally, further laws and policies will hopefully continue to be developed over gene drives in order to regulate the powerful technology. With enough time and research, affected communities and authorities may approve the gene drive strategy and begin implementing it in the near future.
Bibliography
- World Health Organization. 2019. World malaria report 2019. Geneva, Switzerland: World Health Organization.
- World Health Organization. 2020. World malaria report 2020. Geneva, Switzerland: World Health Organization.
- Simoni, A., Hammond, A. M., Beaghton, A. K., Galizi, R., Taxiarchi, C., Kyrou, K., Meacci, D., Gribble, M., Morselli, G., Burt, A., Nolan, T., & Crisanti, A. (2020). A male-biased sex-distorter gene drive for the human malaria vector Anopheles gambiae. Nature biotechnology, 38(9), 1054–1060. https://doi.org/10.1038/s41587-020-0508-1
- Windbichler, N., Menichelli, M., Papathanos, P. A., Thyme, S. B., Li, H., Ulge, U. Y., Hovde, B. T., Baker, D., Monnat, R. J., Jr, Burt, A., & Crisanti, A. 2011. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature [Internet]. 473(7346), 212–215. doi:10.1038/nature09937
- Oberhofer, G., Ivy, T., & Hay, B. A. (2018). Behavior of homing endonuclease gene drives targeting genes required for viability or female fertility with multiplexed guide RNAs. Proceedings of the National Academy of Sciences of the United States of America, 115(40), E9343–E9352. https://doi.org/10.1073/pnas.1805278115
- Centers for Disease Control and Prevention. Insecticide-Treated Bed Nets. Accessed July 15, 2020. Available from: www.cdc.gov/malaria/malaria_worldwide/reduction/itn.html.
- Kahn, Jennifer. 2020. The Gene Drive Dilemma: We Can Alter Entire Species, but Should We? The New York Times Magazine.
- Collins, C. M., Bonds, J., Quinlan, M. M., & Mumford, J. D. (2019). Effects of the removal or reduction in density of the malaria mosquito, Anopheles gambiae s.l., on interacting predators and competitors in local ecosystems. Medical and veterinary entomology, 33(1), 1–15. https://doi.org/10.1111/mve.12327
- Scudellari M. (2019). Self-destructing mosquitoes and sterilized rodents: the promise of gene drives. Nature, 571(7764), 160–162. https://doi.org/10.1038/d41586-019-02087-5
- Bill and Melinda Gates Foundation. Awarded Grants. Accessed 15 July 2020. Available from: www.gatesfoundation.org/How-We-Work/Quick-Links/Grants-Database#q/k=gene%20drive
- Eckhoff PA, Wenger EA, Godfray HC, Burt A. 2017. Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics. Proc Natl Acad Sci U S A [Internet]. 114(2):E255-E264. doi: 10.1073/pnas.1611064114.