By Nikita Jignesh Patel, Neurobiology, Physiology, & Behavior ’22
Author’s Note: Ever since I took BIS2C at UC Davis, I was intrigued as to how our gut microbiome plays such a huge role in our homeostasis beyond just digestion – in particular, the correlation between decreased microbiome diversity and allergies we learned about in the lab fascinated me. I recently stumbled upon the term “gut-brain axis” and was in awe as to how this connection between our gut microbes and our brain even exists, and learned that gut microbiome diversity is implicated in a plethora of mental disorders, from depression and anxiety, to autism. I decided to write this review to share my learning of how the gut microbiome can change the brain and potentially contribute to Autism Spectrum Disorder (ASD), because I feel as if this is not a widely known correlation – even as a physiology major, I never learned about the gut-brain axis in my courses. Moreover, the cause of autism is still widely undefined and the gut microbiome may provide a possible explanation for ASD onset in some individuals. I believe a wide range of students will find this upcoming research interesting, but my intended audience is those who research autism or work with autistic individuals, as it may provide an explanation for ASD and seems to be a likely target for clinical therapy for autism in the future. Above all, I want my readers to take away a better understanding of the gut-brain axis and how its imbalance can be implicated in brain disorders like autism.
Introduction
Autism Spectrum Disorder (ASD) is a lifelong neurodevelopmental disorder characterized by a range of symptoms including difficulty with communication, social interaction, and restricted and repetitive behaviors that present differently in every individual [1]. Although 1 in 54 children are estimated to be on the autism spectrum according to the CDC [2], the etiology of the condition remains poorly understood. Factors including genetics and certain maternal environmental conditions have been identified as potential contributors to the development of ASD in children, but the exact cause is still unknown [3].
A common comorbidity experienced by ASD individuals is gastrointestinal (GI) problems—including abdominal pain, constipation, and diarrhea —as such, autism research is pivoting towards studying the gut microbiome. [1]. Specifically, a link between the composition of the gut microbiome and brain development has been established in recent years — termed the “gut-brain axis”— and it appears to be the future of autism research. This literature review aims to identify the role of the human gut microbiome on the development of autism-like behavior and investigate whether therapies targeting the gut microbiome can be effective clinical treatments for Autism Spectrum Disorder (ASD). The article will first define the differences observed in gut microbiota between autistic and neurotypical individuals, then discuss how these differences in composition may affect brain development, and finally propose clinical implications targeting gut microbiota that appear promising in the treatment and diagnosis of ASD-related behaviors.
Gut Microbiome of ASD Patients Differ From Neurotypical Individuals
The gut microbiomes of Autism Spectrum Disorder (ASD) patients have defining characteristics that significantly differ from those of neurotypical individuals. The human gut microbiome consists of a diverse array of predominantly bacteria but also archaea, eukarya and viruses that possess unique microbial enzymes to aid humans in digestion and also a variety of other physiological functions [4]. The three phyla Firmicutes, Bacteroidetes, and Actinobacteria [5] encompass the majority of bacteria present in the gastrointestinal (GI) tract that aid in these functions. However, an imbalance between the ratio of Firmicutes to Bacteroidetes bacteria is found in autistic individuals when compared to the microbiome composition of neurotypical subjects; in particular, patients with autism tend to have an overexpression of Firmicutes in their gut [6,7]. Other studies have demonstrated an excess of the Clostridium genus in the ASD microbiome [8] as well as an overexpression of the genus Bacilli in the mouths and gut of autistic individuals [7]. While the cause behind this imbalance is unknown, these findings signify a consistent pattern of microbial imbalance in the autistic gut microbiome. In fact, a Random Forest prediction model, a computer algorithm that can classify large sets of data into subgroup “trees” based on data similarity, was able to distinguish ASD children from neurotypical children with a high degree of certainty from just microbiome sequencing data [7], demonstrating the predictability of this dysbiosis by artificial intelligence.
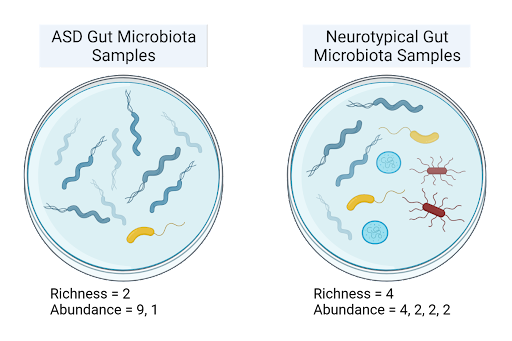
Figure 1: This illustrates the difference between species richness and species abundance. Species richness, a measure of alpha diversity, informs on how many species are present in a sample. Species abundance describes how many organisms of each species are present.
Along with microbial imbalance—termed dysbiosis—autistic children also tend to have a decreased alpha diversity [7,9], which measures mean species diversity, as well as significantly lower gut species richness [7], the number of species present, when compared to age and sex-matched neurotypical children. One study found that for neurotypical children, alpha diversity, species richness, and species abundance all increased between the age groups 2-3 to 7-11; yet for ASD children, no significant development in microbial composition was observed with increase in age [7]. Since autism has been found to slow brain development as children age [9], this reduced development of the microbiome mirrors the altered brain development characteristic of ASD pathophysiology, proposing an association between decreased microbial diversity and autism.
Due to this observed correlation between dysbiosis and ASD, whether gut dysbiosis is truly causal for autism has come into question. In a preliminary study, Sharon et al transplanted fecal microbiota from autistic donors into otherwise germ-free mice (mice with a sterile gut) and observed their offspring’s behavior compared to offspring of mice inoculated with microbiota from neurotypical donors. Notably, mice with the ASD microbiome— characterized by decreased alpha and beta diversity and decreased Bacteroidetes — exhibited behaviors paralleling those of autism, including repetitive behaviors, decreased locomotion and decreased communication [9]. This demonstrated that gut dysbiosis can in fact induce the behavioral deficits observed in ASD. This is significant evidence toward the theory that gut dysbiosis indeed contributes to ASD – an important finding that changes our current understanding of the etiology of autism.
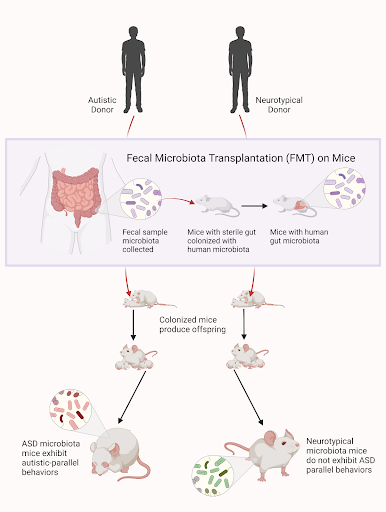
Figure 2: Above is a visual depiction of the study conducted by Sharon & colleagues, where germ-free mice were inoculated with gut microbiota from either autistic or neurotypical donors. Offspring of the mice transplanted with ASD microbiome were shown to exhibit autism-like behavior.
How Microbiota Imbalance Affects Brain Function: The Gut-Brain Axis
Since the microbial dysbiosis found to be common in ASD patients contributes to behavioral deficits, several different mechanisms have been proposed for how the altered microbial environment in ASD patients can affect brain development.
Intestinal permeability
The microbes that line the GI tract provide structural and protective benefits to our intestines, including stimulating epithelial cell regeneration and mucus production by the intestinal walls. When microbial diversity is decreased, the integrity of the intestinal walls may be compromised which can lead to increased intestinal permeability [8]. This may allow for lipopolysaccharide (LPS), a pro-inflammatory endotoxin that is found in gram-negative bacterial cell walls, to escape out of the GI tract and into the bloodstream. Serum levels of LPS are in fact found to be significantly higher in autistic individuals [12]. LPS causes inflammation in the central nervous system (CNS) and is found to impair cognition and motivation in the mouse model. Specifically, implications for impaired continuous attention and curiosity behaviors, along with modulation of other areas of the brain like the central amygdala have been associated with circulating LPS [11]. Therefore, altered intestinal permeability is a possible mechanism by which dysbiosis modulates brain inflammation, a hallmark of autism that is thought to contribute to its behavioral symptoms.
Microbial metabolites
As gut microbes carry out cellular functions inside their human hosts, they also secrete compounds as by-products of metabolism. Two such metabolites are 5AV and taurine, which are secreted by gut Bacteroides xylanisolvens and other bacteria. 5AV and taurine levels are found to be significantly lower in autistic individuals [13,14] as well as mice transplanted with ASD microbiome [9], likely due to dysbiosis. Both 5AV and taurine are gamma-aminobutyric (GABA) receptor antagonists, meaning that lower levels of these circulating microbial metabolites can alter the inhibitory signaling of GABA in the nervous system [9]. GABA regulates various developmental processes in the brain, including cell differentiation and synapse formation, so dysfunction in GABA signalling is thought to account for ASD symptoms [15]. Oral administration of taurine and 5AV in a mouse model of ASD with an altered microbiome is shown to reduce repetitive behavior and increase social behavior, suggesting that the deficiencies in these metabolites may contribute to the behavioral manifestations of autism [9]. There are other microbial metabolite imbalances in autistic children, including dopaquinone, pyroglutamic acid, and other molecules involved in neurotransmitter production. These imbalances affect brain signaling pathways, and therefore could contribute to the behavioral deficits often present in autistic children. Further, these metabolite imbalances correlate with the levels of certain gut bacteria, further emphasizing the link between the gut microbiome and neurological disorders such as ASD.
Clinical Implications for ASD Diagnosis and Treatment
Today, symptoms of autism are alleviated with behavioral and educational therapy, and no pharmaceutical treatment exists [1]. With the knowledge that the gut microbiome significantly differs in autistic individuals and that these differences are shown to interfere with the nervous system, preliminary research has been done on potential diagnostics and pharmaceutical therapeutics for ASD that target dysbiosis in the gut.
Diagnostics
To date, there is no objective laboratory test to detect Autism Spectrum Disorder (ASD) in children, so autism is primarily diagnosed through a doctor’s evaluation of a patient’s behavior and developmental history. However, the ability of a computer program to distinguish the autistic microbiome from the neurotypical microbiome holds potential for use in ASD clinical risk assessments through analysis of the gut microbiome, and subsequent gut health monitoring interventions for those detected to have ASD-like dysbiosis [7]. The strong association between the presence of certain bacterial species in the mouth and bacteria in the gut — in particular the significant positive correlation between saliva Chloroflexi and gut Firmicutes—may suggest possible oral biomarkers to predict gut microbial diversity [6]. In addition, the overexpression of certain bacteria in the gut have been identified to be associated with certain symptoms like allergies and abdominal pain, opening an avenue to improve the diagnosis process of ASD through the inclusion of a more objective, laboratory-based test [6].
Microbiota Transfer Therapy
Microbiota Transfer Therapy (MTT) is an emerging therapy that aims to replace the gut microbiome of ASD individuals with a more diverse, healthy gut microbiome. One form of MTT consists of a two-week oral vancomycin antibiotic treatment, followed by a bowel cleanse using MoviPrep, and then finally an extended fecal microbiota transplant for 7-8 weeks, administered orally or rectally. In a clinical trial involving autistic children, MTT significantly increased gut bacterial diversity 8 weeks after treatment stopped, along with improving GI symptoms (including abdominal pain, indigestion, diarrhea and constipation) measured through the Gastrointestinal Symptom Rating Scale (GSRS). Significant improvements in behavioral ASD symptoms were found post treatment as well, measured through increases from baseline scores on a variety of exams that evaluate social skills, irritability, hyperactivity and communication, among other behaviors [16]. These improvements in microbial diversity and subsequently ASD-related behavior were all found to have been maintained at follow-up study two years later, indicating that MTT is a safe and efficient therapy that has potential to improve ASD outcomes in the long-term [17]. However, further studies on the efficacy of MTT are necessary to establish this connection, as the above study sample was small and most symptoms and improvements were self-reported.
Probiotics
Because imbalances in the microbiome are correlated with autism, direct administration of bacterial cultures using probiotics seems to be a potential approach to treat ASD behavioral symptoms. Probiotic treatment that included a combination of Streptococcus, Lactobacillus, and Bifildobacterium was found to be effective in improving core behavioral symptoms of ASD, specifically adaptive functioning, developmental pathways, and multisensory processing in autistic children with GI symptoms [18]. Probiotics have been shown to improve symptoms of other mood disorders like anxiety and depression, both of which are associated with dysbiosis and the gut-brain axis [8], warranting further research on probiotics as a treatment for ASD. Therapies that target microbial metabolite imbalances in ASD individuals, like 5AV and taurine, may also open an avenue for future autism research [9].
Conclusion
The gut microbiome contributes to the maintenance of much of human physiology, with involvement in not only the digestive system but also the immune system and the brain. Dysbiosis of the gut microbiome has been found to be prevalent in children and adults with Autism Spectrum Disorder (ASD), and this dysbiosis may be linked to the behavioral symptoms observed. Treatments that target the gut microbiome, therefore, serve to be useful in improving behavioral deficits associated with ASD and should be a consideration for future research with more rigorous experimental design.
References:
- Mayo Clinic. Autism Spectrum Disorder. Accessed July 30, 2021. Available from: https://www.mayoclinic.org/diseases-conditions/autism-spectrum-disorder/symptoms-causes/syc-20352928.
- Centers for Disease Control and Prevention. Data & Statistics on Autism Spectrum Disorder. Accessed July 30, 2021. Available from: https://www.cdc.gov/ncbddd/autism/data.html.
- Fattorusso A, Genova L, Dell’Isola G, Mencaroni E, Esposito S. 2019. Autism Spectrum Disorders and the gut microbiota. Nutrients.11(2):521.
- Kho Z, Lal S. 2018.The human gut microbiome—A potential controller of wellness and disease. Frontiers in Microbiology. 9:1835.
- Thursby E, Juge N. 2017. Introduction to the human gut microbiota. Biochemical Journal. 474(11): 1823-1836.
- Kong X, Liu J, Cetinbas M, Sadreyev R, Koh M, Huang H, Adeseye A, He P, Zhu J, Russell H, Hobbie C, Liu K, Onderdonk A. 2019. New and preliminary evidence on altered oral and gut microbiota in individuals with Autism Spectrum Disorder (ASD): Implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients. 11(9): 2128
- Dan Z, Mao X, Liu Q, Guo M, Zhuang Y, Liu Z, Chen K, Chen J, Xu R, Tang J, Qin L, Gu B, Liu K, Su C, Zhang F, Xia Y, Hu Z, Liu X. 2020. Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes. 11(5): 1246-1267
- Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini, A. 2016. Gut microbiota in autism and mood disorders. World Journal of Gastroenterology. 22(1): 361-368.
- Sharon G, Cruz N, Kang D, Gandal M, Wang B, Kim Y, Zink E, Casey C, Taylor B, Lane C, Bramer L, Isern N, Hoyt D, Noecker C, Sweredoski M, Moradian A, Borenstein E, Jansson J, Knight R, Metz T, Lois C, Geschwind D, Krajmalnik-Brown R, Mazmanian S. 2019. Human gut microbiota from Autism Spectrum Disorder promote behavioral symptoms in mice. Cell. 177(6): 1600-1618
- Hua X, Thompson P, Leow A, Madsen S, Caplan R, Alger J, O’Neill J, Joshi K, Smalley S, Toga A, Levitt J. 2013. Brain growth rate abnormalities visualized in adolescents with autism. Human Brain Mapping. 34(2):425-36.
- Haba R, Shintani N, Onaka Y, Wang H, Takenaga R, Hayata A, Baba A, Hashimoto H. 2012. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: Possible role of activation of the central amygdala. Behavioral Brain Research. 228(2):423-31.
- Emenuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, Ucelli di Nemi S, Politi P. 2010. Low-grade endotoxemia in patients with severe autism. Neuroscience Letters. 471(3):162-5
- Ming X, Stein T, Barnes V, Rhodes N, Guo L. 2012. Metabolic perturbance in autism spectrum disorders: a metabolomics study. Journal of Proteome Research. 11(12): 5856-62
- Park E, Cohen I, Gonzalez M, Castellano M, Flory M, Jenkins E, Brown W, Schuller-Levis G. 2017. Is taurine a biomarker in Autistic Spectrum Disorder. Advances in Experimental Medicine and Biology. 975
- Pizzarelli R, Cherubini E. 2011. Alterations of GABAergic signaling in Autism Spectrum Disorders. Neural Plasticity. 2011:297153
- Kang D, Adams J, Gregory A, Borody T, Chittick L, Fasano A, Khoruts A, Geis E, Maldonado J, McDonough-Means S, Pollard E, Roux S, Sadowsky M, Lipson K, Sullivan M, Caporaso J, Brown R. 2017. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 5(1):10
- Kang D, Adams J, Coleman D, Pollard E, Maldonado J, McDonough-Means S, Caporaso J, Krajmalnik-Brown, R. 2019. Long-Term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Scientific Reports. 9(1):5821
- Santocchi E, Guiducci L, Prosperi M, Calderoni S, Gaggini M, Apicella F, Tancredi R, Billeci L, Mastromarino P, Grossi E, Gastaldelli A, Morales M, Muratori F. 2020. Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in Autism Spectrum Disorders: A randomized controlled trial. Frontiers in Psychiatry. 11:550593