By Rhea Bains.
Abstract:
This review comprehensively synthesizes research published within the last five years about a novel immunotherapy for lung cancer, known as a cancer vaccine in situ or intratumoral vaccination. The treatment involves an injection administered directly to the site of the tumor to trigger an immune response in the body for cancer treatment, thereby having the potential to extend the efficacy of other commonly used immunotherapies for lung cancer. Current immunotherapies have been found to not work in all patients and, given that lung cancer is known for high mortality rates, this treatment could potentially save countless lives. The scope of the research reviewed is limited to the two primary subsets of lung cancer vaccines in situ: vaccines containing genetically modified whole cells and vaccines containing modified viruses. It was found that most whole-cell vaccines can be optimized with the concurrent use of existing immunotherapies, but these vaccines have not been proven to improve patient prognoses. This subsequently contributed to the rise of research in the area of virus-based vaccines, which may also require simultaneous use with current immunotherapies to prevent immune-related adverse events. Although each method explored shows promise respectively, it remains unclear which method would be most valuable to isolate as a potential future lung cancer treatment. Many of the trials that show promise were conducted on mouse models, so further human trials of both whole-cell and virus-based vaccines would be beneficial in the quest to expand upon the current capabilities of modern cancer treatment.
Introduction:
Lung cancer is best known for its high rates of mortality, making up for 23% of cancer-related deaths in 2020 alone [1]. Lung cancer’s two main subsets include non-small cell lung cancer and small cell lung cancer, the latter being rarer (15% of all cases) than the former (85% of all cases) [2]. Studied intensively over the past several decades, lung cancer in both its forms has proven a difficult ailment to combat, but extensive progress in treatment has been made. Immunotherapy is considered to be a rising field in potential treatments for advanced cancers. Using the body’s own immune mechanisms to produce an antitumor response is an effective way to improve patients’ prognoses [3]. Leading research institutions work on innovative immunotherapy solutions in the hopes of extending the efficacy of current treatments, which include chemotherapy, radiation, and surgery. Ideally, a new cancer immunotherapy treatment, a vaccine, would help mitigate the common complications brought on by traditional therapies and minimize healthy tissue damage [4].
Recently, there has been a push for the implementation of a cancer vaccine as a vector for future immunotherapies. Because of cancer’s innate ability to evade immune system detection through downregulating antigen (binding molecules) presentation on its cells [5], scientists are looking into a variety of approaches to either mount an immune system response against the tumors actively, using added antigens, or passively, using a nonspecific reaction against the area the tumor cells are located [2-4, 6, 7-9]. Similar to how vaccines evoke a response from the body to fight off infectious diseases, a cancer vaccine, or an intratumoral immunotherapy can be administered at the tumor site to invoke immune mechanisms [10]. An ideal combination of chemical and biological compounds would be administered via an intratumoral immunotherapy injection delivered locally to the site of the tumor (“in situ”), eliciting an immune response. Two components being investigated now fall into the categories of genetically modified virus-based and cell-based vaccines both surmounting an immune response to the tumor [2-4, 6, 7-9]. This review will discuss current research being conducted to evaluate modified viruses and modified whole cells for use in intratumoral immunotherapy, a lung cancer vaccine in situ. Used prior to (neoadjuvant) or after (adjuvant) traditional therapies, advancements in the targets and components of this vaccine could revolutionize lung cancer immunotherapy treatments.
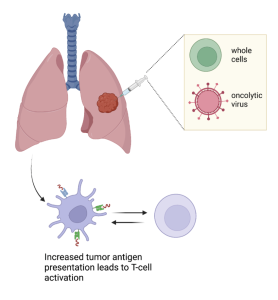
Figure 1: Whole cell and oncolytic virus injections in-situ induce immune response to tumor cells.
Tumor Evasion of Immune System Responses and The Rise of the Cancer Vaccine:
Checkpoint inhibition is a common immunotherapy where patients are administered a compound that commonly blocks interaction between receptors, or specific antigens that bind to the antigens of other cells, found on immune system-derived T-cells and tumor cells, such as the PD-1 and PD-L1 interaction [11]. Normal interaction between the PD-1 and PD-L1 receptors inhibits the T-cell killing of tumor cells in an immune response, which occurs by the interaction of a different pair of cell receptors. An inhibitor of the PD-1 and PD-L1 interaction is known to promote T-cell killing of tumor cells. However, this first-line therapy has been found to only produce a response rate in only 20% of patients [6, 7]. It is unclear why 80% of patients are unable to produce an efficient immune response, but it has been theorized by Lee et al. that these tumors experience under-expressed antigens which limit the response the immune system can produce.
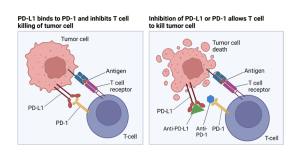
Figure 2: Mechanistic view of T-cell inhibition through PD-1 and PD-L1 interaction.
It is known that some tumors downregulate antigen processing because of low levels of mRNA genes that are essential to signaling the cell to express certain intracellular proteins as antigens. This lowers the amount of antigen expression on the surface of their cells [5]. The human body’s immune cells (including T-cells, dendritic cells, and others) require antigens to effectively recognize tumor cells to bind, and a lack of PD-L1 expression in particular can make tumor cells immune to traditional checkpoint inhibition therapy [6]. Restifo et al. note that while one way to combat this is through administering drugs such as interferon gamma to enhance expression of the low mRNA, this treatment may interfere with the proliferation of T-cells and other immune cells which would counteract effective immune response. Therefore, another method being researched to combat this is using intratumoral immunotherapy to create an immune response that enhances antigen presentation of tumors [4, 6, 9], recruits immune cells [3, 8], and/or induces an inflammatory immune response [7].
Using either cell-based vaccines or virus-based treatments, current research focuses on achieving one or more of the aforementioned results to effectively treat patients with advanced stages of lung cancer. Those who have developed resistance to prior immunotherapeutic treatments or do not initially respond stand to benefit most from vaccination in situ. In combination with other immunotherapies and conventional treatments, this therapy would reduce the recurrence of tumors by building a memory within the immune system. This would transcend the current abilities of treatments like chemotherapy, radiation, and surgery [2], which require multiple treatments in cases of tumor recurrence, each with its own undesirable side effects.
Mechanisms of Modified Whole Cells in Intratumoral Immunotherapy:
The use of modified whole cells in cancer vaccines can be further subdivided into immune cell, tumor cell, and bacterial cell vaccines. The dendritic cell studies mentioned here were conducted in humans while the tumoral and bacterial vaccine studies are mouse-model (murine) based. Each study demonstrated technical success, but there are limitations presented by each of the research groups. Using modified dendritic immune cells to produce an immune response can help improve patient survival rates through increasing antigen presentation for evoked immune responses [6]. Locally injecting a modified dendritic cell injection containing tumor antigens twice and then collecting a tumor biopsy after a seven-day period yielded raised T-cell counts in 8 of 16 patients, so future use of gene-modified dendritic cells along with PD-1 and PD-L1 inhibition could be a promising avenue of therapy. Lee et al. state that tumor cells exhibit low antigen expression, corroborated by Restifo et al., which is why this treatment in concurrence with checkpoint inhibition is ideal to ensure that the best results are achieved. Increased antigen presentation from intratumoral therapy would be used for immune system response to the tumor, and any inhibitory antigens that present could be countered through checkpoint inhibition. Dendritic cells can also be modified to attract other immune cells. These administered whole cells combat cancer’s ability to suppress immune cell activity by recruiting the body’s T-cells to fight off malignancies. Shahrouki et al. conducted a feasibility study and found using dendritic cells in intratumoral immunotherapy to be potentially practical for widespread use, with technical success and minimal complications. However, Shahrouki et al. concurs with the assessment of Lee et al. that the best practice of intratumoral immunotherapy with modified dendritic cells is used in combination with checkpoint inhibitors.
Inactivated whole tumor cells in an intratumoral vaccine can be used to increase antigen expression, primarily when exposed to radiation. Radiation was used in prior studies to inactivate the cells, but Luo et al. explore the possibility that irradiating tumor cells for use in cancer vaccines releases tumor antigens, allowing them to be more easily recognized by the immune cells of the body. The study yielded that irradiated tumor cells produced a substantial immune response in mice, but it is acknowledged that a human trial would be more telling of the efficacy of this treatment.
Bacterial cells can also be used to stimulate an immune reaction, particularly modified E. coli. This bacterium produces a stimulator of interferon genes, which produce interferon gamma, through the STING pathway, activating a cascade of dendritic and T-cells through increased antigen presentation [5, 8]. However, again, this pathway works best when used in combination with a checkpoint inhibitor (atezolizumab), analogous to the findings of Lee et al. and Shahrouki et al, and only 2 of 23 patients experienced stable disease post-treatment. Thus, the markers of increased antigen presentation and immune response in each study are promising, but further human trials need to be conducted with each compound adjacent to checkpoint inhibition to fully understand the efficacy of the treatment.
Mechanisms of Viruses in Intratumoral Immunotherapy:
Discourse in the scientific community suggests that whole-cell vaccines have limited efficacy. Whole cells have shown some success in clinical trials, but further improvements need to be made to enhance it for use in patients [9], and many whole-cell vaccines do not improve overall survival in studies [2]. In fact, although Lee et al. found increased immune response in lung cancer patients, the median survival of the research participants was a mere 3.9 months. As a result of these challenges, some studies have pursued a different method entirely as a result, using select viruses that are injected into tumors via an intratumoral vaccination rather than whole cells.
Using a strategy called gene-mediated cytotoxic immunotherapy (GMCI), a respiratory virus (adenovirus) is administered locally to a tumor site, prior to tumor resection surgery [7]. The oncolytic virus in the form of adenoviruses, herpes simplex viruses, and others only replicate within tumor cells, killing only the cancerous cells in the body [2]. Predina et al. Used an oncolytic virus to selectively infect and kill tumor cells when a secondary drug was added to activate the virus (valacyclovir). This trained the immune system to recognize and kill cancerous cells during a secondary immune response triggered by the inflammation the killing of the tumor cells induces, shrinking the tumor. Post cell death, the inflamed and infected area released tumor antigens that were targeted by the immune system. Predina et al. found that the method elicited increased T-cell activation and more surface antigens, but few patients showed evidence of antibody responses. They hypothesize that the antigen upregulation increased the expression of PD-L1, causing more inhibitory interactions between cancer and T-cells, which could reduce the efficacy of the treatment. Future studies might use this method with checkpoint inhibition to counter the uptake in PD-L1 expression that occurs from increased tumor antigen expression, just as Riese et al., Shahrouki et al., and Lee et al. found in whole-cell vaccine models.
Wang et al. found that although the adenovirus method is effective, two viral injections achieved the best results in a mouse model, rather than just one. The virus would be injected in two parts: the first injection (not intratumoral) would hold whole inactivated tumor cells infected with a modified Newcastle virus, and the second injection would be an intratumoral injection holding just the virus. This virus not only killed tumor cells and upregulated T-cell activation but also increased inflammation and anti-inflammatory responses [9]. Again, this method shows promise but requires further human studies to confirm efficacy; however, Wang et al. provides a solid foundation for future studies on oncolytic viruses in potential intratumoral immunotherapies.
Conclusion:
Intratumoral immunotherapy is a rising field in potential treatment for advanced lung cancer, but there is a wide array of differing opinions about the best mechanism to use to target cancer. While modified cells and viruses have each shown some progress, there are still challenges to be overcome in both areas of research, and consensus has not yet been reached about which method holds the most merit. Both methods may require use with other immunotherapies to maximize success, so current therapies cannot be replaced entirely. There is an array of different types of both cells and viruses that have shown some potential in their own way, so no one method shows significant benefit over the other. In addition, Truong et al. suggest that both methodologies do not account for the typically lower immune system of older patients, who typically make up the demographic suffering from lung cancer. However, current research suggests that in patients with active immune systems (when used with checkpoint inhibitors) cell-based intratumoral vaccines and virus-based vaccines each have the potential to combat low antigen presentation by malignant cells and produce an autologous immune response in patients. With future efforts, perhaps one method of treatment can be isolated and then personalized for individual treatment.
Works Cited:
- Centers for Disease Control and Prevention. An Update on Cancer Deaths in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Division of Cancer Prevention and Control; 2022.
- https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm.
- Truong, Cao-Sang, and So Young Yoo. “Oncolytic Vaccinia Virus in Lung Cancer Vaccines.” Vaccines vol. 10,2 240. 4 Feb. 2022, https://doi.org/10.3390/vaccines10020240.
- Shahrouki, Puja, et al. “Technical Feasibility and Safety of Repeated Computed Tomography–Guided Transthoracic Intratumoral Injection of Gene-Modified Cellular Immunotherapy in Metastatic NSCLC.” JTO Clinical and Research Reports, vol. 2, no. 11, 2021, pp. 100242–100242, https://doi.org/10.1016/j.jtocrr.2021.100242.
- Luo, Lumeng, et al. “Irradiation Increases the Immunogenicity of Lung Cancer Cells and Irradiation-Based Tumor Cell Vaccine Elicits Tumor-Specific T Cell Responses in Vivo.” OncoTargets and Therapy, vol. 12, 2019, pp. 3805–15,
- https://doi.org/10.2147/OTT.S197516.
- Restifo, N. P., et al. “Identification of Human Cancers Deficient in Antigen Processing.” The Journal of Experimental Medicine, vol. 177, no. 2, 1993, pp. 265–72, https://doi.org/10.1084/jem.177.2.265.
- Lee, Jay M., et al. “Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8 + T-Cell Infiltration.” Clinical Cancer Research, vol. 23, no. 16, 2017, pp. 4556–68, https://doi.org/10.1158/1078-0432.CCR-16-2821.
- Predina, Jarrod D., et al. “Neoadjuvant Gene-Mediated Cytotoxic Immunotherapy for Non-Small-Cell Lung Cancer: Safety and Immunologic Activity.” Molecular Therapy, vol. 29, no. 2, 2021, pp. 658–70, https://doi.org/10.1016/j.ymthe.2020.11.001.
- Riese, Richard, et al. “500 SYNB1891, a Bacterium Engineered to Produce a STING Agonist, Demonstrates Target Engagement in Humans Following Intratumoral Injection.” Journal for Immunotherapy of Cancer, vol. 9, no. Suppl 2, 2021, pp. A532–A532, https://doi.org/10.1136/jitc-2021-SITC2021.500.
- Wang, Hui, et al. “Tumor Cell Vaccine Combined with Newcastle Disease Virus Promote Immunotherapy of Lung Cancer.” Journal of Medical Virology, 2023. https://doi.org/10.1002/jmv.28554.
- Hammerich, Linda, et al. “In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf.” Molecular oncology vol. 9,10 (2015): 1966-81.
- https://doi.org/10.1016/j.molonc.2015.10.016.
- Li, Rui, et al. “Inhibition of Granulocytic Myeloid-Derived Suppressor Cells Overcomes Resistance to Immune Checkpoint Inhibition in LKB1-Deficient Non-Small Cell Lung Cancer.” Cancer Research (Chicago, Ill.), vol. 81, no. 12, 2021, pp. 3295–308, https://doi.org/10.1158/0008-5472.CAN-20-3564.